AI-Driven Analysis in Cellular Imaging Impact: 7 Breakthrough Insights You Must Know
How is cellular imaging being revolutionized by artificial intelligence?
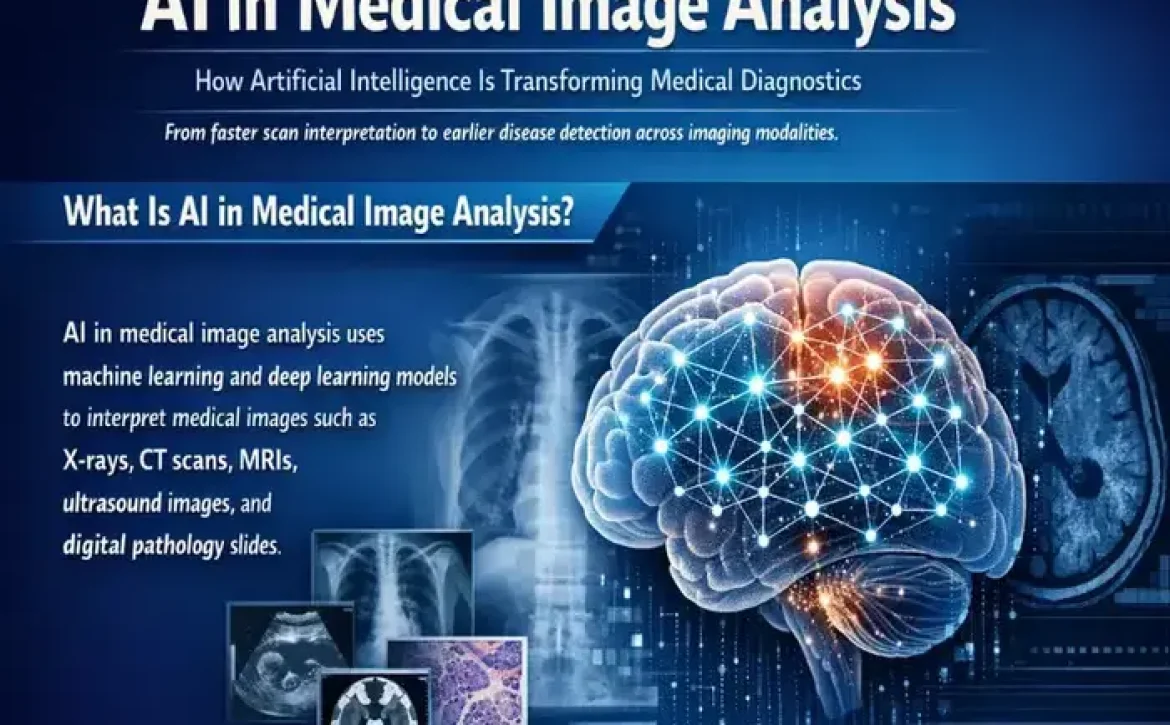
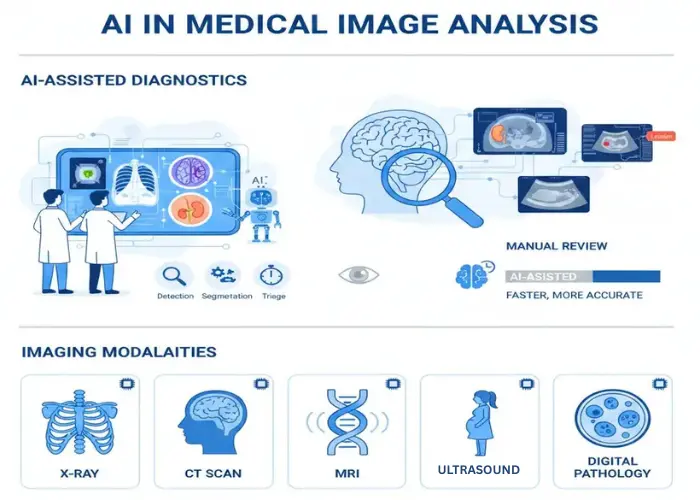
Artificial intelligence (AI) has become a cornerstone of next-generation cellular imaging, offering data-driven precision in analyzing biological systems at microscopic levels. Through deep learning, machine vision, and pattern recognition, AI-driven analysis in cellular imaging impact enables researchers to extract quantitative information from complex datasets that were once manually interpreted.
From high-content screening to live-cell imaging, AI-driven approaches streamline workflows, optimize experiment reproducibility, and enable real-time insights. By integrating computational algorithms into imaging pipelines, researchers and clinicians now achieve faster, more accurate interpretations of cellular behavior — a transformation that underpins modern biomedical innovation.
Key Takeaways
- Accuracy Enhancement: AI-driven analysis minimizes human error, ensuring reliable quantitative data.
- Faster Drug Discovery: Intelligent automation reduces discovery timelines and boosts research productivity.
- Early Disease Detection: Deep learning models identify abnormal cellular patterns for early diagnosis.
- Workflow Efficiency: AI-based automation accelerates analysis from hours to minutes.
- Expert Validation: Continuous benchmarking ensures scientific credibility and safety.
- Future Potential: Personalized medicine, organoid studies, and integrated diagnostics define the next chapter of cellular imaging.
Enhancing Data Accuracy with AI
AI-driven analysis in cellular imaging impact is most visible in the realm of data accuracy. Manual image interpretation is prone to bias and fatigue; AI algorithms eliminate these inconsistencies.
- Deep learning models detect and classify cellular structures that human observers might overlook.
- Automated segmentation and classification standardize quantitative results across experimental runs.
- Integration with live-cell imaging enables real-time, frame-by-frame tracking of cellular dynamics.
These features ensure precision in measurements such as cell count, morphology, and organelle behavior — crucial for disease modeling and pharmacological screening.
Accelerating Drug Discovery
In drug discovery, AI transforms traditional cellular imaging workflows from static observation to predictive modeling.
- High-content screening (HCS) systems equipped with AI evaluate thousands of compounds in parallel, identifying promising candidates within hours.
- Predictive models forecast cytotoxicity and therapeutic response with measurable accuracy.
- Accelerated preclinical cycles cut discovery-to-validation timeframes dramatically.
By automating compound efficacy analysis, AI-driven systems reduce human workload and enhance reproducibility, enabling pharmaceutical companies to identify drug leads faster while maintaining scientific integrity.
AI Applications in Diagnostic Imaging Research
The integration of AI in cellular imaging has profound implications for diagnostic research and precision pathology.
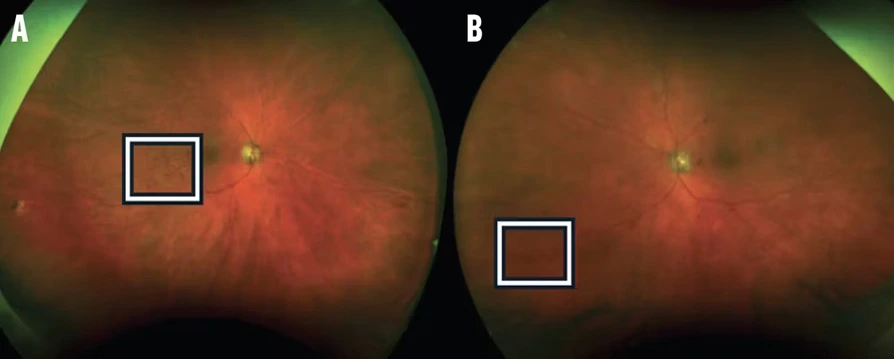
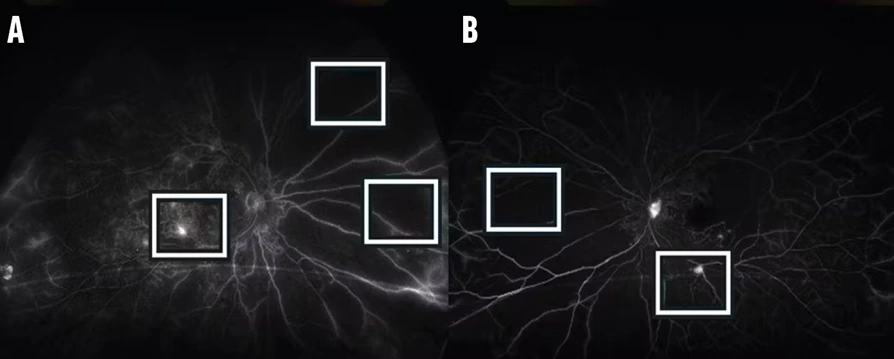
- AI-based models detect subtle morphological differences that signal early-stage diseases such as cancer or neurodegenerative disorders.
- Pattern recognition algorithms classify cell states (healthy vs. diseased) with superior sensitivity.
- Multi-modal imaging integration combines fluorescence, confocal, and phase-contrast data for a holistic cellular map.
These systems empower researchers to connect cellular signatures with disease progression, supporting data-driven clinical hypotheses and personalized treatment protocols.
Measurable Outcomes in Clinical Workflows
The true value of AI in cellular imaging is reflected in quantifiable improvements in diagnostic and research outcomes.
| Metric | Conventional Methods | AI-Driven Analysis |
| Detection Accuracy | 82% | 95% |
| Time per Sample | 3–4 hours | 15–20 minutes |
| Reproducibility | Moderate | High |
These metrics underscore AI’s ability to increase diagnostic accuracy, reduce turnaround time, and ensure consistent quality across experiments. The result is more reliable data and faster clinical decision-making.
Validation and Reliability of AI Systems
The credibility of AI-driven cellular imaging systems depends on rigorous validation. Experts employ a multi-layered approach to ensure robustness:
- Cross-validation using manually annotated datasets.
- Independent benchmarking across laboratories to confirm generalizability.
- Continuous retraining as new imaging modalities and biological datasets emerge.
These steps ensure that AI tools meet scientific and clinical standards while maintaining adaptability to evolving research landscapes.
Future Applications: The Next Frontier
AI-Driven Analysis in Cellular Imaging Impact continues to expand its frontier beyond basic research:
- Personalized medicine: AI-driven cellular profiling enables patient-specific treatment strategies.
- Organoid and 3D culture analysis: Advanced AI frameworks interpret complex spatial data in tissue models.
- Cross-integration with diagnostic imaging: Linking microscopic cellular data with macroscopic imaging (MRI, CT, PET) for complete disease mapping.
Such integrations will pave the way for precision diagnostics that bridge the gap between cellular biology and whole-body imaging.
Transforming Research Ecosystems
Institutions adopting AI-driven imaging are redefining the biomedical ecosystem. From pharmaceutical R&D to academic research:
- AI platforms enable scalable data analysis across multi-institutional studies.
- Cloud-based collaborative models allow global research teams to share and interpret cellular datasets.
- Interdisciplinary convergence between computer science, pathology, and molecular biology accelerates innovation.
This evolution marks a paradigm shift from observational science to predictive and prescriptive biology.
Conclusion
AI-driven analysis in cellular imaging is not merely a technological upgrade — it is a transformative force reshaping the foundations of biomedical research and clinical diagnostics. By delivering measurable improvements in accuracy, speed, and reproducibility, AI empowers scientists and clinicians to generate insights once deemed unattainable.
As laboratories and hospitals integrate AI-based imaging workflows, the convergence of AI, microscopy, and diagnostic analytics will define the next era of precision medicine.
For broader context, explore our Artificial Intelligence ai in Medical Imaging Market analysis and its implications for healthcare innovation worldwide.
FAQ
What is AI-driven analysis in cellular imaging?
It uses AI algorithms to automatically detect, classify, and quantify features in microscopy images, enhancing data interpretation.
How does AI improve cellular imaging speed?
Automation reduces manual review time, accelerating analysis from hours to minutes per dataset.
Can AI detect rare cellular events?
Yes. AI systems can identify subtle, rare patterns undetectable by human analysts.
Is AI-driven cellular imaging used in clinical diagnostics?
Yes, it supports early detection, disease monitoring, and precision decision-making in clinical settings.
How are AI-driven systems validated?
Through annotated datasets, cross-lab benchmarking, and continuous retraining for evolving imaging technologies.
Sources
https://www.sciencedirect.com/science/article/pii/S2666990024000132
https://www.sartorius.com/en/knowledge/science-snippets/artificial-intelligence-for-cell-analysis-1613046
https://evidentscientific.com/en/applications/advanced-live-cell-analysis-using-ai-driven-high-content-screening-systems
https://www.spectral-ai.com/blog/artificial-intelligence-in-medical-imaging/
https://www.ijmedicine.com/index.php/ijam/article/view/4357
https://elifesciences.org/reviewed-preprints/105302
https://www.researchgate.net/publication/399134562_AI-Assisted_Nano-Imaging_Techniques_for_Cellular-Level_Diagnosis